41 orbital diagram for oxygen
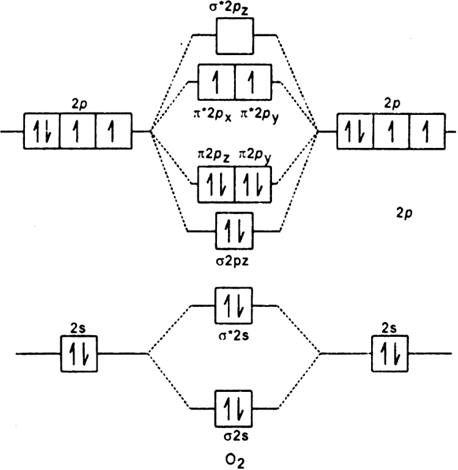
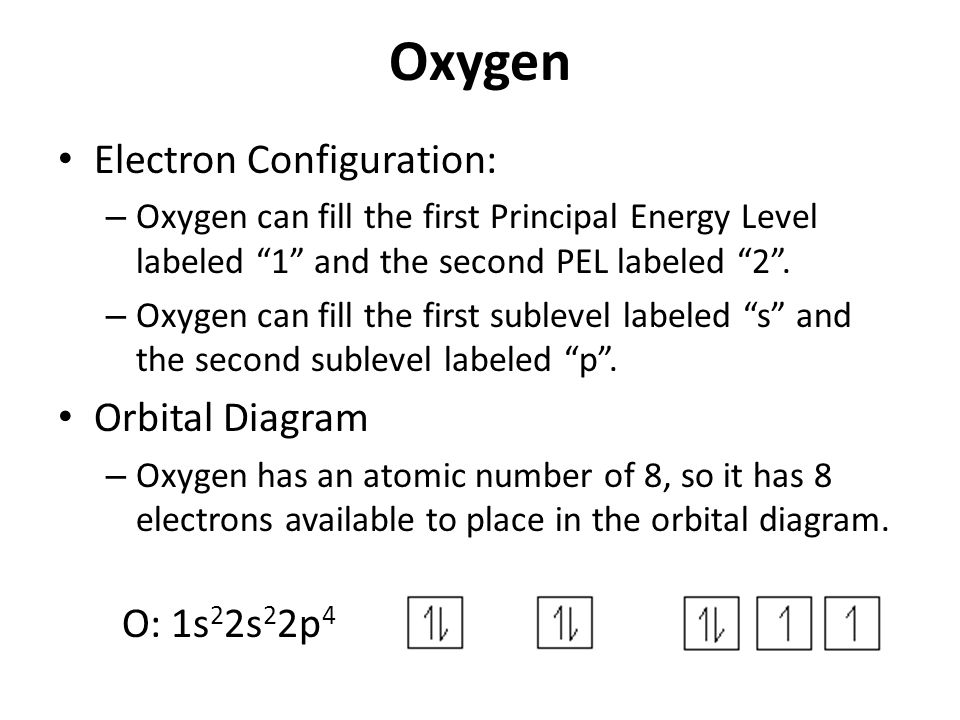
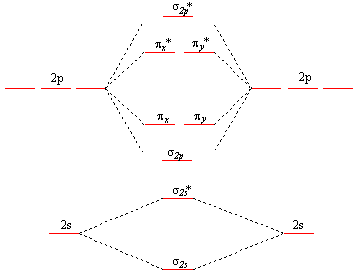
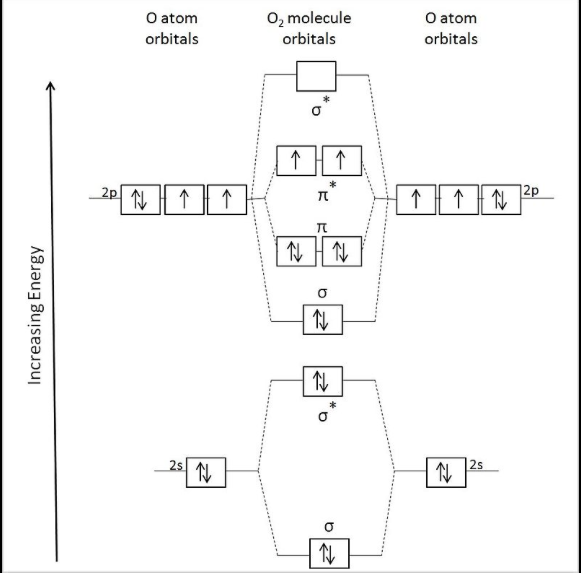
Explain the formation of O2 molecule using molecular class ... We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as - How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Solved Complete this valence molecular-orbital diagram for ... Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed. Question: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.

Orbital diagram for oxygen
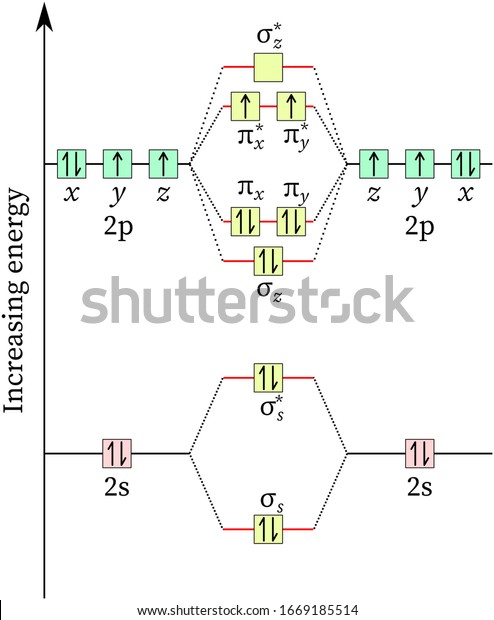
CHEM 492 - Molecular Orbitals of Dioxygen According to the MO diagram, oxygen has a bond order of 2. According to the MO diagram, oxygen has a bond order of 3. The Lewis structure for oxygen shown is accurately represented by the MO diagram below. Based on the completed MO diagram, it is clear that pi-type overlap is a weaker interaction than coaxial overlap. Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. orbital diagram of oxygen - menuswitch.com The orbital diagram for a ground-state oxygen atom is 1s (up down) 2s (up down) 2p (up down, up, up) Which of the following is the electron configuration of an excited state of an oxygen Therefore, the electronic configuration of O In case of these elements, the order of energy levels of `sigma`2pxm, `pi` 2px and `pi`2py is reversed.
Orbital diagram for oxygen. Molecular Orbital Theory - Purdue University The only orbitals that are important in our discussion of molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. Sodium(Na) electron configuration and orbital diagram Sodium(Na) is the 11th element in the periodic table and its symbol is ‘Na’. This article gives an idea about the electron configuration of sodium and orbital diagram, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in . 8 - Drawing Molecular Orbital Diagrams — Flux Science Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
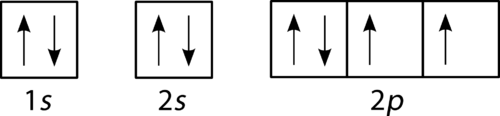
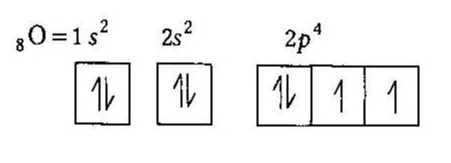
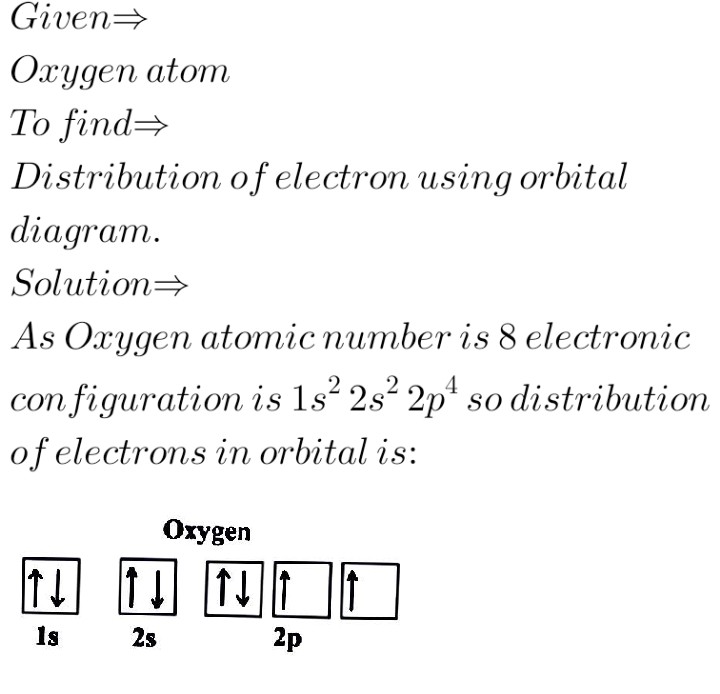
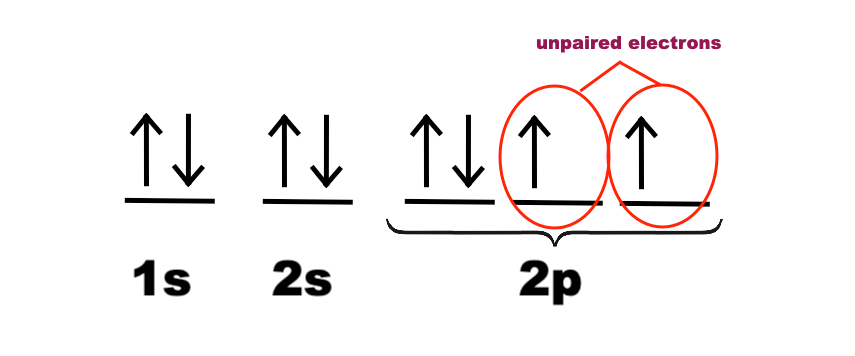
How To Draw Molecular Orbital Diagram Of O2 Molecular orbital diagram for oxygen gas (o2).fill from the bottom up, with 12 electrons total.bonding order is 2, and it is paramagnetic.sigma2s(2),sigma2s*. The first major step is understanding the difference between two. The sides of the diagram just refer back to where those molecular orbitals came from, with dotted lines to guide you from ... What Is The Orbital Diagram Of Oxygen? [Comprehensive Answer] Oxygen is usually found as a diatomic gas. Therefore, we write it as O2. How do you draw a molecular orbital diagram for oxygen? Starts here14:38Molecular orbital energy diagram of Oxygen(O2) molecule. - YouTubeYouTubeStart of suggested clipEnd of suggested clip35 second suggested clipAs the unpaired electrons are there in pi star pi star 2px. Oxygen Orbital diagram, Electron configuration, and Valence ... The orbital diagram for Oxygen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Oxygen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest four electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Oxygen atom is shown below- Electron Configuration Worksheet - Easy Hard Science Oxygen O is element 8 with 8 electrons when it’s neutral. The 1s orbital is full, the 2s orbital is full, and there are 4 electrons to draw in the 3 boxes in the 2p orbital. As per Hund’s Rule, there would be 3 arrows pointing up in the 2p orbital and 1 pointing down. So there is a pair of electrons in the first box of the 2p only.
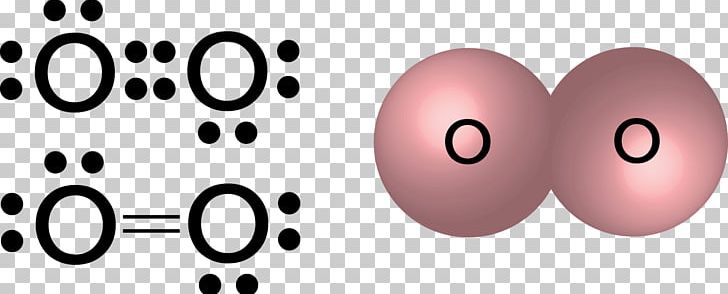
Oxygen(O) electron configuration and orbital diagram This is clearly shown in the figure of the orbital diagram of oxygen. Orbital Diagram for Oxygen (O) Oxide ion (O 2-) electron configuration Ground state electron configuration of oxygen is 1s 2 2s 2 2p x2 2p y1 2p z1. This electron configuration shows that the last shell of oxygen has six electrons. Molecular orbital diagram - Wikipedia Carbon and each oxygen atom will have a 2s atomic orbital and a 2p atomic orbital, where the p orbital is divided into p x, p y, and p z. With these derived atomic orbitals, symmetry labels are deduced with respect to rotation about the principal axis which generates a phase change, pi bond ( π ) [26] or generates no phase change, known as a ... Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
8.4 Molecular Orbital Theory – Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram ( Figure 7.7.9 ). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center.
Give the orbital diagram for an atom of oxygen. | Study.com The orbital diagram is also one way of representing the electron configuration. Answer and Explanation: 1 Become a Study.com member to unlock this answer! Create your account View this answer The...
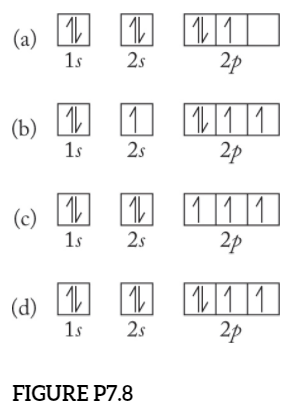
Solved Which orbital-filling diagram represents the ground ... Which orbital-filling diagram represents the ground state of oxygen? A. [ [He] 신 N 2p 치어 B. [He] 신 소 소 2p 25 C. [He] 个个 2s 신 소 소 2p D. [He] _ 28 솨 신 소 2p 치 ; Question: Which orbital-filling diagram represents the ground state of oxygen? A.
Molecular Orbital (MO) Diagram for O2(-) - YouTube When two oxygen atoms overlap, the sigma(2p) molecular orbital is LOWER in energy than the pi(2p) orbitals. This different from Nitrogen, where it's the othe...
PDF Draw the orbital diagram for oxygen Draw the molecular orbital diagram for oxygen molecule. Learning objective to draw, interpret and convert between Lewis (Kekule), condensed and bond line structures Note: The revision of the general chemistry in the Sections 1.3 - 1.6 is integrated into the learning goal above for the chemical organ ¢ Nica in the Sections 1.7 and 1.8.
Draw molecular orbital diagram of O2 or N2 with magnetic ... Draw molecular orbital diagram of O 2 or N 2 with magnetic behavior and bond order. Medium Solution Verified by Toppr As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b −N a ]/2=[10−6]/2=2.
Electron Configuration for Oxygen (O) - UMD Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be ...
Draw the molecular orbital energy diagram for oxygen ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds ...
How do yo write the orbital diagram for oxygen? | Socratic The electron configuration for oxygen is: 1s22s22p4 This video will walk you through the step of writing orbital diagram. The video uses Kr as an example, but the process is exactly as the same as what you need to do for oxygen. Hope this helps! Answer link
What Is The Molecular Orbital Diagram Of O2 ... What is the bond order of O2 -? 2 O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. How do you draw a molecular orbital diagram for O2? 0:003:38Molecular Orbital (MO) Diagram of O2 - YouTubeYouTube. How many electrons are in bonding orbitals in O2? 1: Molecular Orbital Energy-Level Diagrams for O2.
What is the orbital diagram of oxygen? - findanyanswer.com In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. Click to see full answer
Orbital Diagram For Oxygen - The 32 Best Images, Videos ... The 32 best 'Orbital Diagram For Oxygen' images and discussions of March 2022. Trending posts and videos related to Orbital Diagram For Oxygen!
40 o2+ molecular orbital diagram - Wiring Diagrams Manual Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
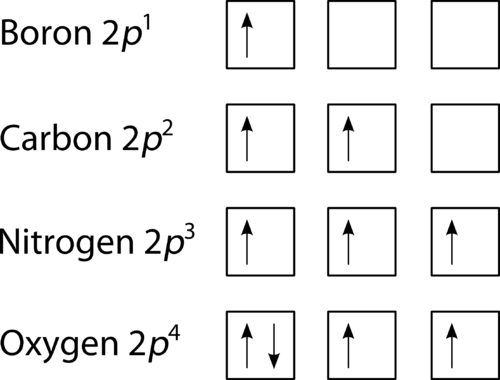
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2).
orbital diagram of oxygen - menuswitch.com The orbital diagram for a ground-state oxygen atom is 1s (up down) 2s (up down) 2p (up down, up, up) Which of the following is the electron configuration of an excited state of an oxygen Therefore, the electronic configuration of O In case of these elements, the order of energy levels of `sigma`2pxm, `pi` 2px and `pi`2py is reversed.
Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
CHEM 492 - Molecular Orbitals of Dioxygen According to the MO diagram, oxygen has a bond order of 2. According to the MO diagram, oxygen has a bond order of 3. The Lewis structure for oxygen shown is accurately represented by the MO diagram below. Based on the completed MO diagram, it is clear that pi-type overlap is a weaker interaction than coaxial overlap.





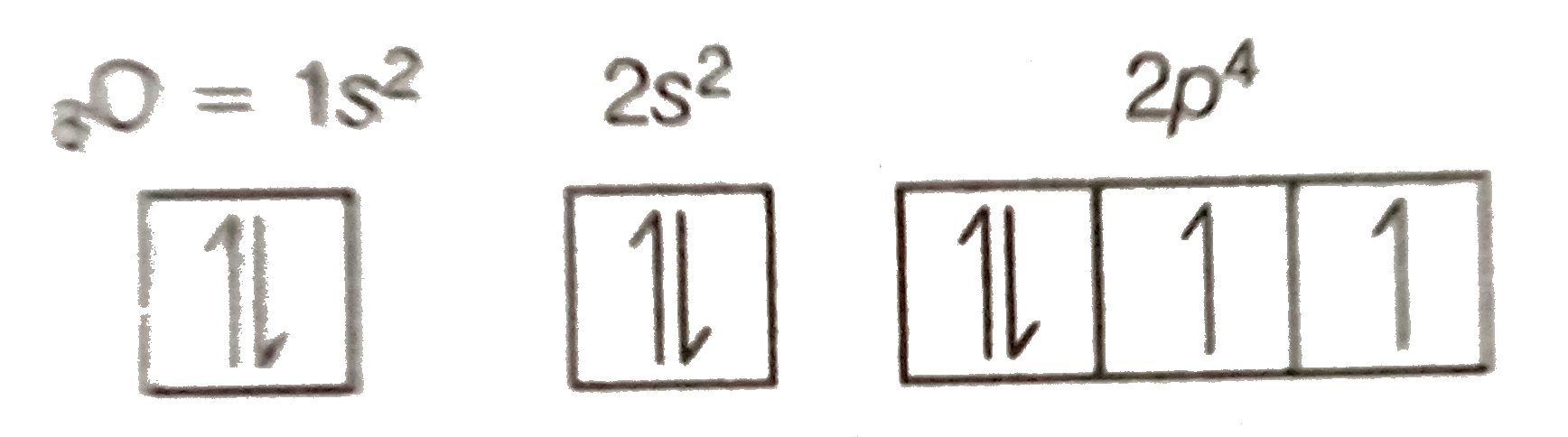


















0 Response to "41 orbital diagram for oxygen"
Post a Comment